|
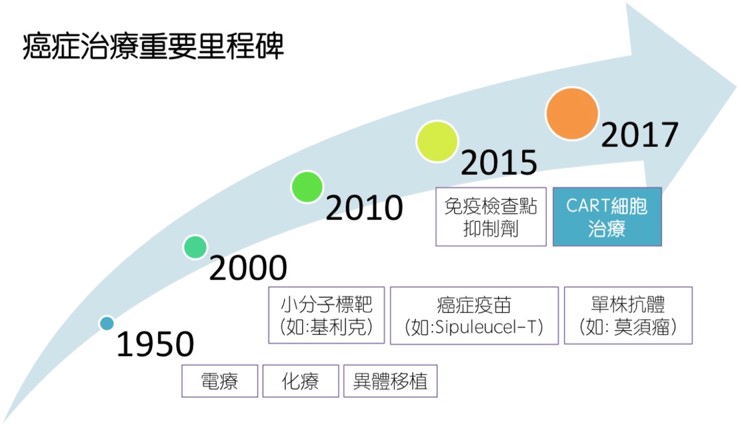
1. The Future of Cancer Treatment: Chimeric Antigen Receptor-T cell therapy Standard treatments for cancer include surgery, chemotherapy, targeted therapy, and radiation therapy. Recently immunotherapy (treatment enhancing the patient's own immune ability to fight against tumor cells) has become the fifth pillar of cancer treatment. Immunotherapy has three main areas of development: tumor vaccines, immune checkpoint inhibitors, and cell therapy. For cell therapy, the most promising one is chimeric antigen receptor T cell therapy (CAR-T cell therapy). CAR-T cell therapy is a kind of T cell infusion therapy, which uses genetic engineering to make the patients’ own T cells capable of recognizing and killing tumor cells. Although most CAR-T cell therapy is still in the clinical trial stage and focused mainly on hematological malignancy, some patients receiving CAR-T have achieved complete response and long-term remission. As a result, currently there is a global effort in research and development of CAR-T therapy. Since the U.S. Food and Drug Administration (FDA) approved the clinical trials of CAR-T cells in treating acute lymphoblastic leukemia (ALL) and lymphoma, many international pharmaceutical companies have devoted to the research and development of CAR-T. In 2017, Novartis' CTL019 Tisagenlecleucel (trade name Kymriah) was launched, followed by Axicabtagene ciloleucel (Kite Pharma). Both CAR-Ts are used to treat B cell ALL or lymphoma expressing CD19 antigen. It can be expected that more CAR-T therapy will be available in the future.
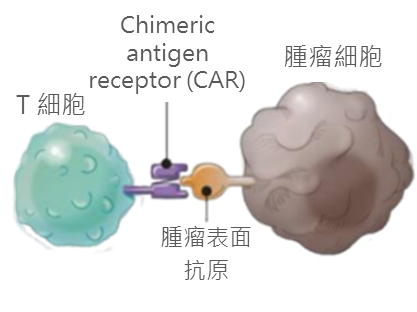
2. Manufacturing Protocols of Chimeric Antigen Receptor-T cell therapy The human immune system can identify its own cells and then attack and eliminate cells invaded by foreign pathogens. Although cancer cells express mutated proteins, patients’ own immune cells are often unable to recognize these cells. For CAR-T cells production, antibody fragments are designed that could recognize specific surface antigens of cancer cells, and genetic material coding the fragments are sent into T cells by viruses, in order to make T cells with tumor recognition capabilities. The basic process consists of the following steps: Isolation: Isolate the patient's T cells by standard leukapheresis Gene editing: Using viruses to deliver genetic material into T cells, so that the cell membranes of T cells express antibodies that can recognize tumor-specific molecules Expansion: T cells are first activated and expanded by using magnetic beads coated with specific stimulatory signal proteins Reinfusion: CAR-T cells with tumor antigen recognition ability are given back to the patient Monitoring: Because the infusion of a large number of T cells will cause cytokine release syndrome (CRS), it is necessary to pay attention to the vital signs of the patient after the infusion and deal with any possible complications at any time.
4. Development of CAR-T Cell Therapy in Taipei Veterans General Hospital The Medical Research Department has established the Cell Therapy Innovation Research and Development Center. A CAR-T cell therapy team, including physicians and researchers from clinical as well as research departments, was sent up. Team members meet regularly to discuss the latest developments in cancer immunotherapy. Since CAR-T cell therapy is becoming the trend of cancer therapy, which is also the key development goal in our hospital, a lot of resources have been invested by hospital in order to make the treatment more accessible for patients. CAR-T therapy requires a group of highly professional expertise including physicians, nurses, and technicians, as well as a well-equipped environments. Currently we are working on training personnel and establishing required facilities. The CAR-T cell therapy team will work to develop this state-of-the art therapy in the future.
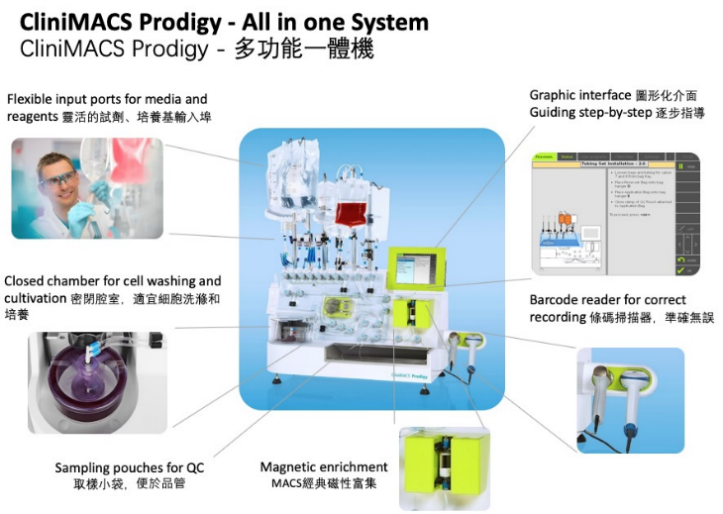
5. Progress of CAR-T Cell Therapy in Taipei Veterans General Hospital After obtaining the official permission of the Ministry of Health and Welfare, we have produced the first CAR-T cell product at our hospital on December 28, 2022 by using the CliniMACS Prodigy system for the isolation, activation, transduction, and expansion of T/CAR-T cells. CAR-T cells quality monitoring and safety testing were also carried out during manufacturing to ensure safety, purity, potency, and consistency. The CAR-T cell manufacturing process was completed on January 9, 2023, and the product safety test including hepatitis B surface antigen detection were complete thereafter. The CAR-T cell production was finalized and reinfused to patient in February 2023. |
Last Modified: